
Dietmar Tietz
Section on Macromolecular Analysis
Laboratory of Theoretical and Physical Biology
Building 10, Room 6C-101, NICHD, National Institutes of Health
Bethesda, Maryland 20892, USA
Protein-conjugated meningitis vaccines have been developed by John Robbins, Rachel Schneerson and co-workers [1,2] for the immunization of small children, the main target group of bacterial meningitis. The physical characterization of these immunogens has been difficult, since their surface net charge is high and since the particles have a relatively large size which is in the range of intact viruses. Moreover, the size distribution of vaccine particles varies continuously over a wide range (polydisperse distribution) due to the randomizing steps in their method of preparation. When such samples are subjected to one-dimensional agarose electrophoresis, they exhibit an uninterpretable smear rather than a pattern of distinct zones (Fig. 1). However, when a second dimension is added to the separation, the results become interpretable: Distribution patterns are obtained which are characteristic for each immunogen. The patterns are digitized by scanning and then stored as computer images (Fig. 2). Using the computer programs ElphoFit [3] and GelFit [4], the size and charge distributions of these patterns as well as a number of other parameters are determined [5]. In particular, program ElphoFit is used to evaluate the gel electrophoretic data and to standardize the gel on the basis of the extended Ogston model ([6,7], Fig. 2 of [3]). Output of ElphoFit is then transferred to computer program GelFit which transforms the original images to a rectangular coordinate system of particle radius and free mobility as it is exemplified in Fig. 3. GelFit also computes frequency distributions of size and free mobility classes depicted by pseudocolors (Fig. 3 of [5]).
Acknowledgments
The author thanks Dr. Andreas Chrambach (SMA, LTPB, NICHD, NIH) for support, Dr. Akram Aldroubi and Dr. Michael Unser (Biomedical Engineering and Instrumentation Program, NCRR, NIH) for providing computer program GelFit, and Dr. Benes Trus (Division of Computer Research and Technology, NIH) for instruction in image processing. Vaccine samples were kindly provided by Dr. Rachel Schneerson (LDMI, NICHD, NIH).

Fig. 1:Gel patterns of meningitis immunogens at different agarose concentrations, using a one-dimensional submarine electrophoresis apparatus [8]. The samples yield an uninterpretable smear, although electrophoretic conditions are appropriate.
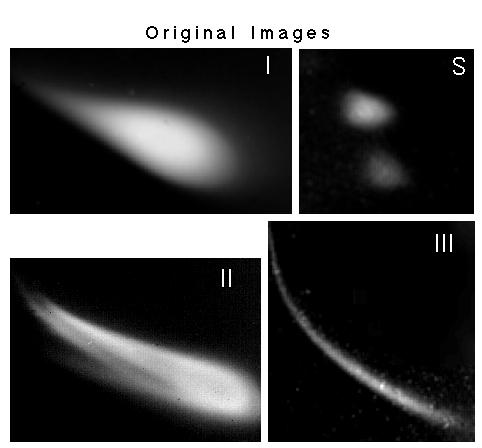
Fig. 2:Two-dimensional gel patterns of meningitis immunogens (I to III) and of hydrophilic polystyrene size standards (S). The origin of electrophoresis lies outside the pictures. First dimension (top to bottom): 0.15 % agarose (SeaPlaque), 3 V/cm, second dimension (left to right): 0.8 % agarose, 1.5 V/cm. Electrophoresis in phosphate buffer pH 7.2 using a modified [9] 2-D submarine electrophoresis apparatus [10], staining: Coomassie Blue R 250. The vaccines produce characteristic gel patterns which depend on the nature of the sample. The samples consist of Haemophilus influenzae, type b, capsular polysaccharide crosslinked to tetanus toxoid (Panels I and II) or P2 protein (Panel III). The pattern of II can be interpreted as the composite of three subpopulations as has been shown in Figs.6 and 7 of [5] and may derive from combining different vaccine batches.
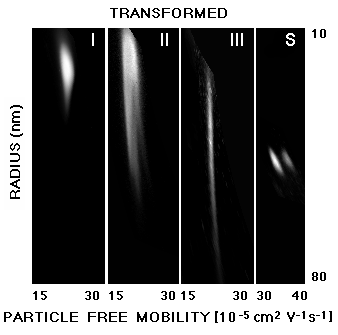
Fig. 3:Patterns of Fig. 2 which have been transformed from the original curvilinear to a rectangular coordinate system of particle size and free mobility (related to surface net charge density). Vaccines II and III which were effective immunogens have a considerably larger size distribution than sample I (not effective). The immunogens I and II have a much larger variation in free mobility than III, since protein P2 is well defined, whereas tetanus toxoid is a mixture of many components. It should be noted that the 2-D electrophoresis used here achieves results similar to O'Farrell's technique, but relies on a different principle: Predominant charge- (1st dimension) and predominant size-separations (2nd dimension) are achieved by using gels with low and relatively high agarose concentrations under non-denaturing conditions. By applying a mathematical approach [9,3] based on the extended Ogston model [6,7], one can distinguish the separation effects due to particle size and charge. The investigated particles are in the size range of viruses (10,000 to 2,000,000 kD). O'Farrell-type gels, by comparison, are typically used for much smaller proteins and protein fragments (10 to 500 kD) and achieve charge-separation by isoelectric focusing and size-separation by denaturing SDS electrophoresis.
References
[1] Schneerson, R., Barrera, O., Sutton, A., and Robbins, J.B.: Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361-375.
[2] Devi, S.J.N., Robbins, J.B., and Schneerson, R: Antibodies to poly[(2->8)-a-N-acetylneuraminic acid] and poly [(2->9)-a-N-acetylneuraminic acid] are elicited by immunization of mice with Escherichia coli K92 conjugates: Potential vaccines for groups B and C meningococci and E. coli K1. Proc. Natl. Acad. Sci. USA 1991, 88, 7175-7179.
[3] Tietz, D.: Analysis of one-dimensional gels and two-dimensional Serwer-type gels on the basis of the extended Ogston model using personal computers. Electrophoresis 1991, 12, 28-39.
[4] Aldroubi, A., Unser, M., Tietz, D., and Trus, B.: Computerized methods for analyzing two-dimensional agarose gel electropherograms. Electrophoresis 1991, 12, 39-46.
[5] Tietz, D., Aldroubi, A., Schneerson, R., Unser, M., and Chrambach, A.: The distribution of particles characterized by size and free mobility within polydisperse populations of protein-polysaccharide conjugates, determined from two-dimensional agarose electropherograms. Electrophoresis 1991, 12, 46-54.
[6] Ogston, A.G.: The spaces in a uniform random suspension of fibres. Trans. Faraday Soc. 1958, 54, 1754-1757.
[7] Rodbard, D. and Chrambach, A.: Unified theory of gel electrophoresis and gel filtration. Proc. Natl. Acad. Sci. USA 1970, 65, 970-977.
[8] Serwer, P.: Improved procedures for controlling the voltage gradient and temperature during electrophoresis in submerged horizontal gel slabs. Electrophoresis 1983, 4, 227-231.
[9] Tietz, D. and Chrambach, A: Computer-assisted evaluation of polydisperse 2-dimensional gel patterns of polysaccharide-protein conjugate preparations with regard to size and net charge. Electrophoresis 1989, 10, 667-680.
[10] Serwer, P.: Two-dimensional agarose gel electrophoresis without gel manipulation. Anal. Biochem. 1985, 144, 172-178.
Elektrophorese Forum '91, B. J. Radola ed., Technische Universität München, Freising-Weihenstephan, Germany, pp. 452-456 (1991).